PART 3: DOMAIN KNOWLEDGE – BASICS ON AQUAPONICS
This post is a continuation of Basic deep learning with aquaponics, Part 2.
Introduction
It is recommended that one should read the entirety of Small-scale aquaponic food production by FAO.
One important aspect of doing data science is having domain knowledge. Without domain knowledge, one can not interpret the data in a meaningful way. In this post we will discuss the basics of aquaponics.
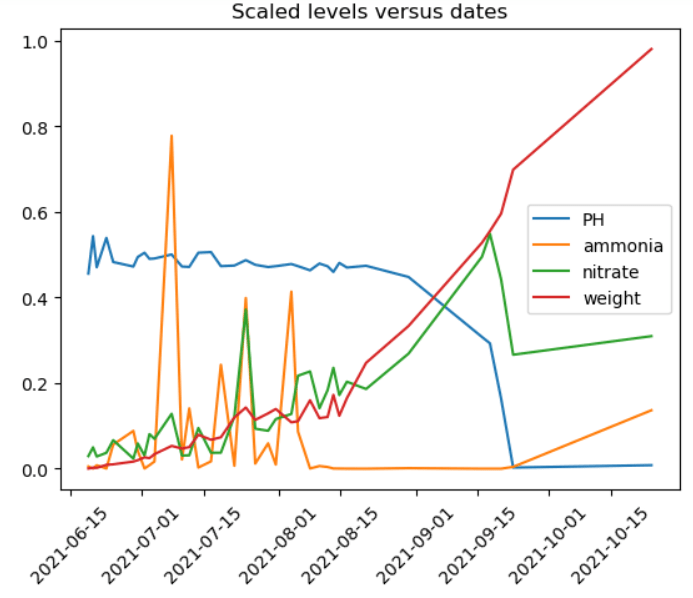
A healthy aquaponic system requires balancing certain parameters such as temperature, dissolved oxygen, ammonia, nitrites, nitrates, and pH. The goal is minimizing inputs of raw materials and maximizing the outputs such as fish and plants. It’s a balancing act that attempts to replicate nature. Recall the plot of various scaled measurements from PART 2 showing basic interaction in an aquaponic system.
In an aquaponic system, the fish, plants, and bacterias coexist in a recirculated aquatic system. Ammonia is released into the system from the fish metabolic waste and the decomposition of uneaten fish feed. The ammonia is then turned into nitrites by ammonia-oxidizing bacteria such as Nitrosomonas, and the nitrites are then turned into nitrates by nitrite-oxidizing bacteria such as Nitrobacter. This process is called nitrification. Finally, the ammonia, nitrites, nitrates, and other nutrients are then consumed by the plants, thus filtering the water to a safer level for the fish.
From the equations we observe that both genera are aerobic (consume oxygen). Furthermore, ammonia-oxidizing bacterias release hydrogen ions, which would lower the pH level. There is also a trace amount of nitric acid produced once the nitrates become readily available
Nitric acid is a strong acid, so it can quickly lower the pH once it accumulates over time. A certain amount of buffering would be needed to resist the change in the pH value. We will discuss more of buffering later below.
Beside photosynthesis, plants need six macronutrients in large amounts and small amount of various micronutrients. The six macronutrients consist of nitrogen, phosphorous, calcium, magnesium, and sulphur. The micronutrients consist of iron, manganese, boron, zinc, copper, and molybdenum. Most of these nutrients are obtained from the nitrification process and decomposition of uneaten fish feed. Iron is usually supplemented as chelated iron, and potassium and calcium are usually obtained when buffering with potassium carbonate and calcium carbonate. The uptake of these nutrients is best when the pH is slightly acidic.
What is pH?
Let’s first looking into the pH level. Water molecules often dissociate into hydrogen ions and hydroxide ions. However, hydrogen ions would immediately binds to another water molecule producing hydronium:
In pure water, the amount of hydronium ions, measured in moles per liter, is approximately 1.0 x 10-7 at room temperature (20-25 celsius). The amount of hydroxide is the same in pure water. For most aqueous solution, the molarity of hydronium ions range from 1.0 x 10-14 to 1.0 x 100. Now, the pH of any solution is defined to be the negative logarithm of its molarity of hydronium ions
This concentration of hydronium (or hydrogen ions) determine whether an aqueous solution is acidic (pH < 7), neutral (pH = 7), or basic (pH > 7). Though there are three definitions of acids and bases, we will use Arrhenius as it is most most relevant to aquaponics. According to Arrhenius, acids donate hydrogen ions H+ while bases donate hydroxide ions OH– (or equivalently, accept hydrogen ions). Both types of ions hop from one water molecule to the next, thus causing high electrical conductivity when the pH is not neutral.
Balancing an aquaponic system
The main parameters that we will monitor in an aquaponic system are: water temperature, dissolved oxygen (DO), ammonia, nitrites, nitrates, pH, and fish weight. The fish, plants, and bacterias each have different tolerance levels for the aforementioned parameters. Below is a table from FAO displaying recommended target ranges for each parameter. Here, KH is a measure of alkalinity, which we will discuss later below.

Balancing the pH
A sustainable system requires the pH to be in the range 6-8. The range 7-8 is optimal for fish and microbes (Timmons et al. 2002, Tyson et al. 2008), while the range 6-7 is optimal for plants to uptake important nutrients (Resh 2013); see also the nutrient availability chart below from FAO:

A high pH value above 7.5 can lead to nutrient deficiencies such as iron, phosphorous, and manganese. A pH value below 6 can inhibit the nitrifying bacterias from converting the ammonia to nitrates. Of course, pH value well beyond the neutral level will be unsustainable for the three organisms.
The pH of an aquaponic system is highly affected by nitrification process (as mentioned in the introduction), and also by the fish weight. A densely population of fish would release a large amount of ammonia and carbon dioxide, which is then combined with water to produce a weak acid called carbonic acid and thus lowering the pH.
Carbonic acid has chemical interactions with carbonates and bicarbonates that will help buffer or resist changes in pH. Below, we will discuss alkalinity, which is a measure of carbonates and bicarbonates.
Alkalinity
Carbonate hardness or alkalinity is the amount of carbonates and bicarbonates dissolved in water. It is measure in mg of calcium carbonates per liter, labeled KH. In general, we want the range of KH to be 60-140 mg/L. When nitric acid is formed from the nitrification process, the pH is lowered. As it’s a strong acid, the pH can be dramatically lowered quickly if left unchecked. The hydrogen ions from the nitric acid binds and remain bound with carbonates and bicarbonates. The hydrogen ions binding with the carbonates will yield bicarbonates, and the binding with the bicarbonates will yield the weak carbonic acid. The bicarbonates chemical reaction with nitric acid has the form
The pH remains stable and there are more nitrates for the plants – it’s a win-win situation. Typically we buffer using salts such as calcium carbonate and potassium carbonate, which would also provide essential nutrients calcium and potassium to the plants – more wins!
Dissolved oxygen and temperature
Dissolved oxygen (DO) is the most essential parameter for the plants, fish, and nitrifying bacterias. Among the three, the fish would need the most DO (5-8 mg/L). It follows that if enough DO is provided for the fish, then the DO for the plants and bacterias would be plentiful. DO can be provided to the system using water movement (with a venturi tube) and aerators.
Different variety of fish and plants have different temperature tolerance; however, a range of 18-30 C is preferable. Even though warmer temperature is more beneficial for the nitrifying bacterias, it can have devastating consequences for other parameters. As temperature increases, the solubility of oxygen decreases. Temperature increases would also invigorate the metabolic rate of the fish and the bacterias as mentioned above, which would further decreases the dissolved oxygen level. There will also be fluctuation in DO as the plants (and algae) respire nightly to generate energy.
When DO is low, certain anaerobic bacterias will proliferate and lead to undesirable effects. For example, denitrification may happen in which nitrates are converted back to molecular nitrogen and nitrous oxide, which is a potent greenhouse gas.
Ammonia, ammonium, nitrates, and nitrites
Ammonia is released into the system mainly from the fish metabolic waste and decomposition of uneaten feed. Ammonia can exist in two forms, unionized (NH3) and ionized (NH4+). The nitrifying bacterias convert the ammonia to nitrites and then to nitrates. Both ammonia and nitrites are incredibly toxic to fish. Even a small amount can be stressful for the fish over a long period of time. Hence, in an optimal system all of the ammonia and nitrites should be converted to nitrates.
In an acidic condition, there are plenty of hydrogen ions to convert ammonia to its less toxic ionized form called ammonium:
The two forms of ammonia together is called total ammonia nitrogen (TAN), with around 10% being unionized ammonia. Any water testing kit will actually be testing TAN. As the pH and temperature increase, the TAN ratio heads towards the more toxic form of ammonia and overloads the nitrifying bacterias. Hence, it’s beneficial to keep environment slightly acidic.
In the next post, we will perform time-series exploration on Pond 4.
Leave a reply to Basic deep learning with aquaponics, Part 4 – Sophelen Research Cancel reply